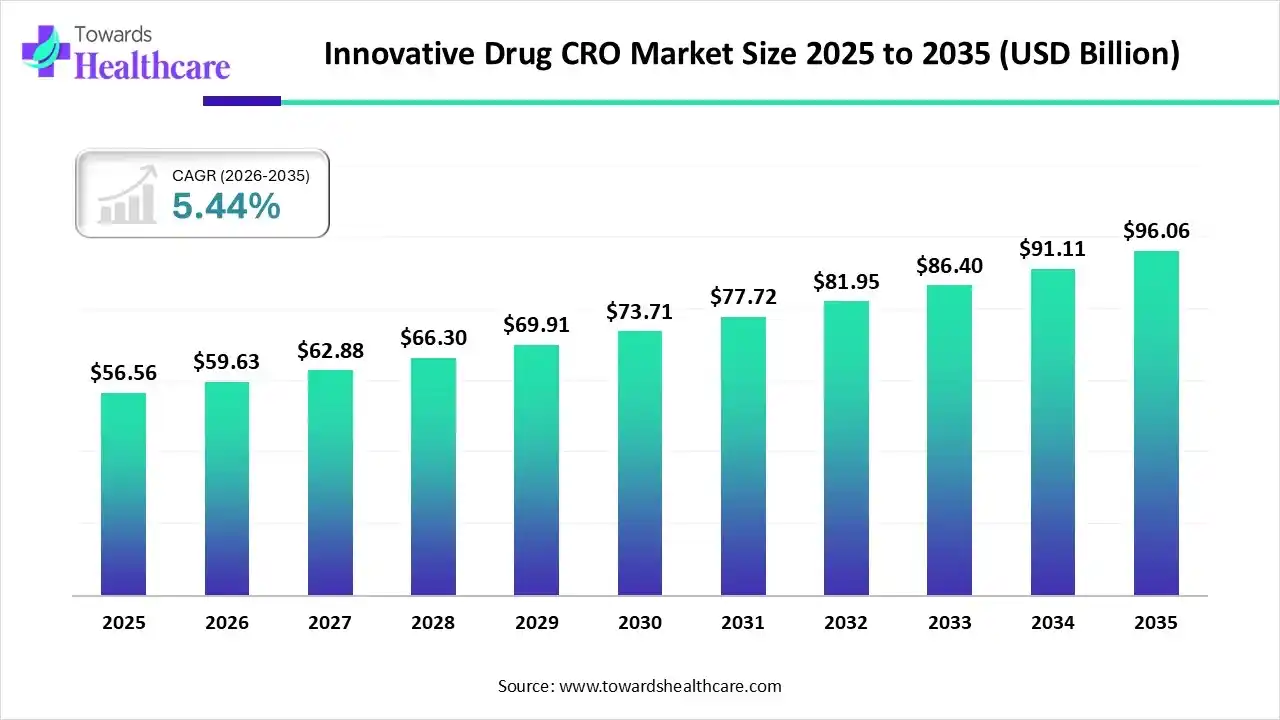
Innovative Drug CRO Market to Soar USD 96.06 Billion by 2035, Expanding at 5.44% CAGR
The global innovative drug CRO market size is valued at USD 56.56 billion in 2025 and is predicted to hit around USD 96.06 billion by 2035, rising at a 5.44% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
Ottawa, Nov. 28, 2025 (GLOBE NEWSWIRE) -- The global innovative drug CRO market size is calculated at USD 59.63 billion in 2026 and is expected to reach around USD 96.06 billion by 2035 growing at a CAGR of 5.44% for the forecasted period, driven by the increasing R&D costs and growing innovations.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/6344
Key Takeaways
- North America dominated the market with approximately 40% share in 2024.
- Asia-Pacific is expected to be the fastest-growing region in the innovative drug CRO market during the forecast period.
- By service type, the clinical development services segment held a major revenue of approximately 50% share of the market in 2024.
- By service type, the laboratory services segment is expected to be the fastest-growing in the market during the forecast period.
- By therapeutic area, the oncology segment held a major revenue of approximately 35% share of the market in 2024.
- By therapeutic area, the rare diseases segment is expected to be the fastest-growing in the market during the forecast period.
- By end-user, the pharmaceutical companies segment held a major revenue of approximately 45% share of the market in 2024.
- By end-user, the biotechnology companies segment is expected to be the fastest-growing in the market during the forecast period.
- By business model, the full-service CROs segment held a major revenue of approximately 50% share of the market in 2024.
- By business model, the specialty/niche CROs segment is expected to be the fastest-growing in the market during the forecast period.
- By technology/platform adoption, the electronic data capture (EDC) & eclinical platforms segment held a major revenue of approximately 35% share of the market in 2024.
- By technology/platform, the artificial intelligence/machine learning platforms segment is expected to be the fastest-growing in the market during the forecast period.
What is the Innovative Drug CRO?
The innovative drug CRO market is driven by increasing clinical trial complexities, R&D costs, and the adoption of advanced technologies. Innovative drug CRO refers to the CROs focused on novel and advanced therapies and providing specialized services from preclinical research to clinical trials with laboratory testing and regulatory expertise.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What are the Major Drivers in the Innovative Drug CRO Market?
The growing oncology and rare disease pipeline is the major driver in the market. The growing disease and its unmet needs are increasing their innovation, which is increasing the demand for these services for outsourcing. Additionally, regulatory complexities, expanding startups, and growing clinical trials are other market drivers.
What are the Key Drifts in the Innovative Drug CRO Market?
The market has been expanding due to the growing acquisitions to launch and enhance the use of CRO services.
- In September 2025, the successful acquisition of Symbiance, which is a data-driven, technology-enabled Contract Research Organization (CRO), consisting of expertise in clinical data services and operations, along with services like pharmacovigilance, medical monitoring, project management, and medical writing, was announced by ACL Digital.
- In May 2025, the acquisition of Paris-based ILIFE Consulting was announced by UK-based Comac Medical Group, which will support Comac’s aim of becoming a pan-European full-service CRO with expertise in rare diseases, oncology, and early-phase clinical trials.
What is the Significant Challenge in the Innovative Drug CRO Market?
Limited availability is the major challenge in the market. This is due to a lack of skilled professionals in clinical, regulatory, and lab areas, which decreases their dependence rates. Moreover, data security concerns, dependence on sponsor budget, and stringent regulatory requirements are other market limitations.
Regional Analysis
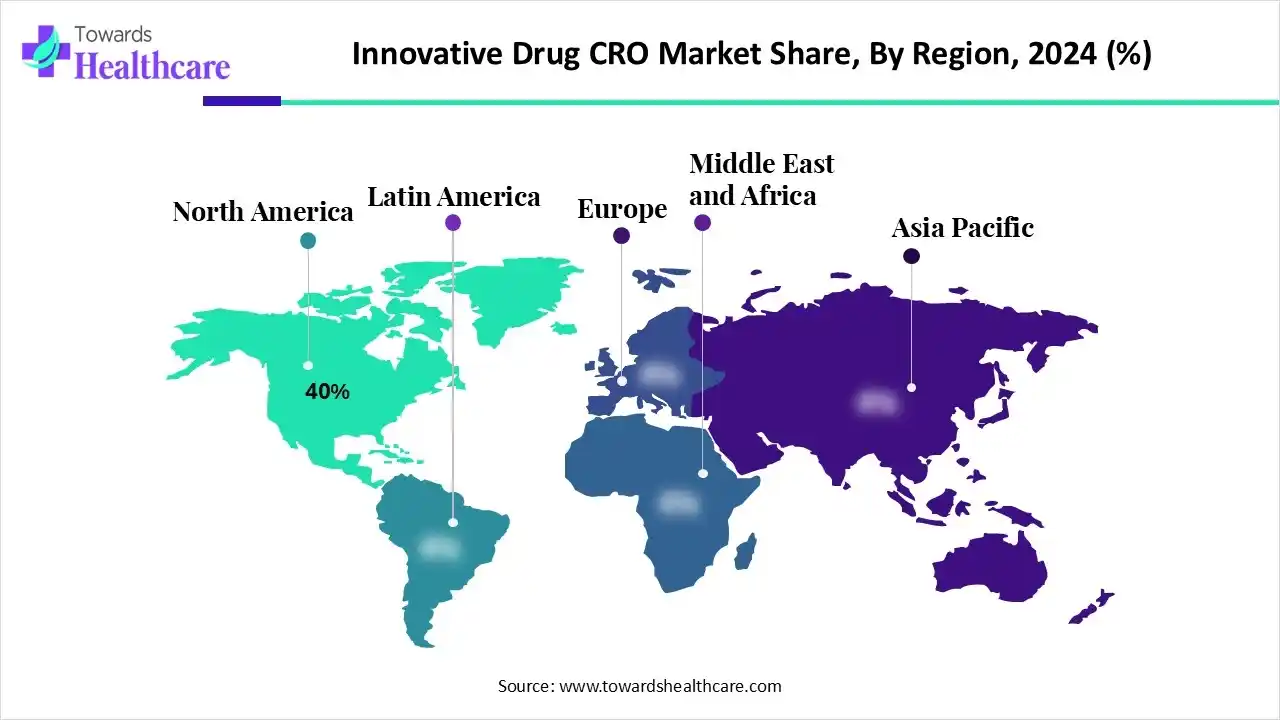
Why did North America Dominate the Innovative Drug CRO Market in 2024?
In 2024, North America captured the biggest revenue share of 40% in the market, due to the presence of advanced industries. The growth in the R&D investments and stringent regulations are encouraging the use of innovative drug CROs. The growing drug development and clinical trials also increased their demand, where the companies developed advanced technologies, which contributed to the market growth.
What Made Asia Pacific Grow Rapidly in the Innovative Drug CRO Market in 2024?
Asia Pacific is expected to host the fastest-growing market during the forecast period, due to expanding industries, CRO infrastructure, and innovative therapies. Similarly, the growing clinical trials and government support are increasing the use of these services. Additionally, the companies are developing advanced platforms, which are promoting the market growth.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
Segmental Insights
By service type analysis
Why Did the Clinical Development Services Segment Dominate in the Innovative Drug CRO Market in 2024?
By service type, the clinical development services segment led the market with approximately 50% share in 2024, due to its expertise in therapeutic areas. They also accelerated the clinical trial and reduced the operational cost. Additionally, they also offer experienced regulatory teams that ensure regulatory compliance.
By service type, the laboratory services segment is expected to show the fastest growth rate during the predicted time, due to growing demand for specialized testing. The growing precision medicine and expanded cell and gene therapies are also increasing their demand. They are also being used for decentralized trials.
By therapeutic area analysis
Which Therapeutic Area Type Segment Held the Dominating Share of the Innovative Drug CRO Market in 2024?
By therapeutic area, the oncology segment held the dominating share of approximately 35% share of the market in 2024, due to growth in the R&D investments. The services also helped to deal with complex and lengthy trials. Furthermore, the growth in the development of novel therapies and precision medicine has also increased their use.
By therapeutic area, the rare diseases segment is expected to show the highest growth during the predicted time, due to growing R&D activities. The growing regulatory incentives and therapies are also increasing their demand for their expertise and decentralized approach.
By end-user analysis
How the Pharmaceutical Companies Segment Dominated the Innovative Drug CRO Market in 2024?
By end-user, the pharmaceutical companies segment led the market with approximately 45% share in 2024, due to growth in the clinical pipeline and R&D investments. This increased the use of CRO services to accelerate their clinical trials and reduce developmental costs, which contributed to the market growth.
By end-user, the biotechnology companies segment is expected to show the fastest growth rate during the predicted time, due to expanding therapies. Moreover, the growing investments and outsourcing trends have also increased their demand. The growing startups are also leveraging their services.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
By business model analysis
What Made Full-Service CROs the Dominant Segment in the Innovative Drug CRO Market in 2024?
By business model, the full-service CROs segment held the largest share of approximately 50% in the market in 2024, driven by their end-to-end capabilities. They also provided enhanced operational efficiency and reduced the developmental cost. This increased their use by various industries.
By business model, the specialty/niche CROs segment is expected to show the highest growth during the upcoming years, due to growing interest in niche therapeutic areas. This, in turn, is increasing the demand for specialized labs and advanced technologies, which is increasing the dependence of these CROs.
By technology/platform adoption analysis
Why Did the Electronic Data Capture (EDC) & eClinical Platforms Segment Dominate in the Innovative Drug CRO Market in 2024?
By technology/platform adoption, the electronic data capture (EDC) & eclinical platforms segment led the market with approximately 35% share in 2024, as they act as a central hub for clinical data. They also provided real-time data access and enhanced data quality and accuracy, which increased their use.
By technology/platform adoption, the artificial intelligence/machine learning platforms segment is expected to show the fastest growth rate during the upcoming years, as they support the development of precision medicines. They also accelerate drug development, provide predictive analytics, and help in data management.
Browse More Insights of Towards Healthcare:
The global drugs market size recorded US$ 1688.14 billion in 2025, set to grow to US$ 1790.05 billion in 2026 and projected to hit nearly US$ 3034.63 billion by 2035, with a CAGR of 6.04% throughout the forecast timeline.
The global drug discovery market size is calculated at USD 65.88 billion in 2024, grew to USD 71.96 billion in 2025, and is projected to reach around USD 160.11 billion by 2034. The market is expanding at a CAGR of 9.24% between 2025 and 2034.
The global drug screening market size was estimated at US$ 6.15 billion in 2023 and is projected to grow to US$ 10.34 billion by 2034, rising at a compound annual growth rate (CAGR) of 4.84% from 2024 to 2034.
The drug-induced dyskinesia market size is calculated at US$ 418 million in 2024, grew to US$ 436.2 million in 2025, and is projected to reach around US$ 627.5 million by 2034. The market is expanding at a CAGR of 4.34% between 2025 and 2034.
The global drug-loaded fat emulsion market size is calculated at US$ 2.34 billion in 2024, grew to US$ 2.62 billion in 2025, and is projected to reach around US$ 7.25 billion by 2034. The market is expanding at a CAGR of 12.04% between 2025 and 2034.
The global drug discovery platforms market size is calculated at USD 186.24 million in 2024, grew to USD 211.26 million in 2025, and is projected to reach around USD 635.45 million by 2034, growing at a 13.44% CAGR from 2025 to 2034.
The global drug discovery as a service market size is calculated at US$ 21.3 in 2024, grew to US$ 24.32 billion in 2025, and is projected to reach around US$ 79.82 billion by 2034. The market is expanding at a CAGR of 14.17% between 2025 and 2034.
The global drug repurposing market size is calculated at US$ 636.95 million in 2025, grew to US$ 730.96 million in 2026, and is projected to reach around US$ 2506.64 million by 2035. The market is expanding at a CAGR of 14.76% between 2026 and 2035.
The global drug designing tools market size is calculated at US$ 3.4 in 2024, grew to US$ 3.7 billion in 2025, and is projected to reach around US$ 7.86 billion by 2034. The market is expanding at a CAGR of 8.73% between 2025 and 2034.
The drug-device combination products market size was estimated at US$ 150.3 billion in 2023 and is projected to grow to US$ 337.81 billion by 2034, rising at a compound annual growth rate (CAGR) of 7.64% from 2024 to 2034.
The global topical drugs CDMO market size is calculated at US$ 46.32 in 2024, grew to US$ 51.62 billion in 2025, and is projected to reach around US$ 136.71 billion by 2034. The market is expanding at a CAGR of 11.43% between 2025 and 2034.
Recent Developments in the Innovative Drug CRO Market
- In October 2025, an integration of AI technologies into Contract Research Organization (CRO) services to enhance access to high-quality reagents and robust analytical tools was announced by Smart Launch.
- In April 2025, oncology models and research services were launched by Powered Research, which is a leading preclinical CRO specializing in non-GLP models.
Innovative Drug CRO Market Key Players List
- Medpace
- Charles River Laboratories
- WuXi AppTec
- PPD (Thermo Fisher)
- Eurofins Scientific
- PRA Health Sciences
- Bioclinica
- Frontage Laboratories
- Pharmaron
- CTI Clinical Trial & Consulting Services
- SGS Life Sciences
- Celerion
- Kendle International
- Pharmaceutical Product Development (PPD)
Segments Covered in The Report
By Service Type
- Preclinical Services
- In Vivo Studies
- In Vitro Studies
- Toxicology Studies
- ADME/DMPK Studies
- Clinical Development Services
- Phase I Trials
- Phase II Trials
- Phase III Trials
- Phase IV/Post-Marketing Studies
- Regulatory & Safety Services
- Regulatory Submissions & Consulting
- Pharmacovigilance & Drug Safety
- Laboratory Services
- Bioanalytical Services
- Genomics/Biomarker Services
- Central Laboratory Services
- Others
By Therapeutic Area
- Oncology
- Cardiovascular Diseases
- CNS / Neurology
- Infectious Diseases
- Autoimmune/Immunology
- Metabolic Disorders
- Rare Diseases
- Others
By End-User
- Pharmaceutical Companies
- Biotechnology Companies
- Academic & Research Institutions
- Government & Public Health Organizations
By Business Model
- Full-Service CROs
- Specialty/Niche CROs
- Hybrid CROs
By Technology/Platform Adoption
- Artificial Intelligence/Machine Learning Platforms
- Electronic Data Capture (EDC) & eClinical Platforms
- Remote Monitoring & Decentralized Trial Technologies
- Digital Biomarker & Wearable Integration Platforms
- Cloud-Based Data Management & Analytics
By Region
North America
- U.S.
- Canada
- Mexico
- Rest of North America
South America
- Brazil
- Argentina
- Rest of South America
Europe
-
Western Europe
- Germany
- Italy
- France
- Netherlands
- Spain
- Portugal
- Belgium
- Ireland
- UK
- Iceland
- Switzerland
- Poland
- Rest of Western Europe
- Eastern Europe
- Austria
- Russia & Belarus
- Türkiye
- Albania
- Rest of Eastern Europe
Asia Pacific
- China
- Taiwan
- India
- Japan
- Australia and New Zealand,
- ASEAN Countries (Singapore, Malaysia)
- South Korea
- Rest of APAC
MEA
- GCC Countries
- Saudi Arabia
- United Arab Emirates (UAE)
- Qatar
- Kuwait
- Oman
- Bahrain
- South Africa
- Egypt
- Rest of MEA
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/6344
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

